1.1 Current risk assessment practices
1.1.1 What is Risk Analysis related to food contaminants?
Risk Analysis is a systematic way to fully assess risks related to toxic compounds present in the diet, to get transparency into the complexity of the problem and to address uncertainties or knowledge gaps. It allows risk managers to make their decisions about measures to be taken to reduce possible risks and it facilitates the communication about risk to the general public. Risk analysis consists of three components:
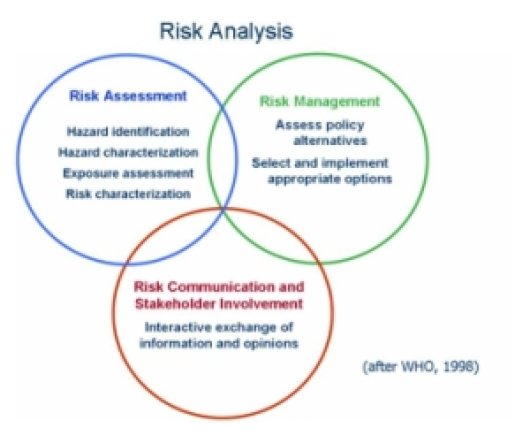
- Risk assessment (link to WP5 module)
- Risk management (link to WP5 module)
- Risk communication (link to WP5 module)
In this module we will focus our attention on current risk assessment practices.
1.1.2 Risk Assessment
In a food context, risks involve potential impacts on consumers when ingesting foods. Possible hazards present in foods are infectious microorganisms, and chemicals occurring as contaminants (e.g. cleaning compounds, pesticides) or physical agents (e.g. glass).

Even though efforts are taken to minimise hazards from happening, food safety can not be guaranteed for 100% and hazards can occur. Risk assessment follows a structured approach to estimate the risk and to obtain insight in the factors that influence the risk in both a positive and negative way. A risk can be estimated in absolute terms (e.g. number of consumers getting ill per year from eating certain foods) or in relative terms (e.g. comparing the safety of one food with that of another). The aim of a risk assessment is eventually to minimize the risk that health problems will occur in a certain population.
Risk assessment consists of 4 steps:
- Hazard identification: this step deals with the identification of agents (biological, chemical or physical) that are capable of causing an adverse health effect.
- Hazard characterisation: this step has to do with the qualitative and/or quantitative evaluation of the nature of the adverse health effects. For both chemical agents and biological or physical agents, a dose-response assessment should be performed if the data are obtainable.
- Exposure assessment: this step deals with the qualitative and/or quantitative evaluation of the degree of intake likely to occur. This step will be mentioned in more detail in the section entitled: Dietary Exposure Assessment.
- Risk characterisation: this step integrates the information gathered in the previous 3 steps into an estimation of the adverse effects likely to happen in a certain population, including related uncertainties.
Sources:
- Codex Alimentarius website
- WHO website
- EUFIC website
- J. L. Jouve, M. F. Stringer and A.C. Baird Parker (1998) “Food Safety Management Tools”, April, ILSI
1.1.3 Risk assessment limitations
Current risk assessment procedures do not result in a fixed final answer. Some of the limitations include:
- Lack of information on environmental conditions and processes that affect the properties of the contaminant such as transformation, degradation, temperature, pH, etc.
- Uncertainty in extrapolating toxicity study data between species (e.g. using the outcome of animal testing to predict the effect on people) and within species (e.g. different sensitivity of people)
- Databases used to assess the risks in different countries are not comparable, as well as the methodology used to assess the risks
- Large information gaps about the effects of contaminant mixtures that may have synergistic, magnifying or other effects
- Large information gaps about the specific mechanisms and processes affecting functions and organs within the body, how these interact, and how they might be affected by a contaminant
- Existing models looking only at point estimates of both exposure and effect
- Existing models address only single compound exposure, and are therefore not able to address those situations where multi-compound analysis is imperative (e.g. when looking at chemicals with a common mechanism of action or in risk-benefit issues)
Source: http://contamsites.landcareresearch.co.nz/limitations_of_risk_assessment.htm
